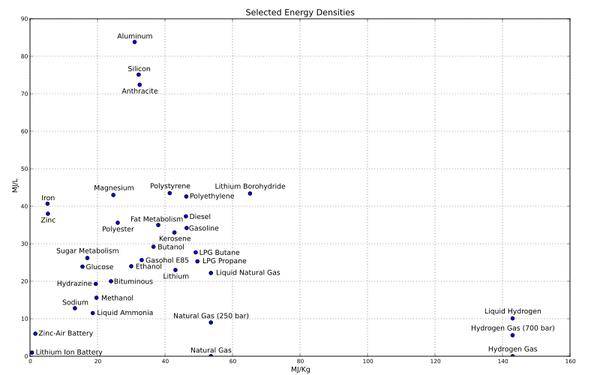
What exactly is limiting the battery capacity?For this problem, we can see this: battery capacity = energy density x battery volume. The battery size naturally want to do on how to do, energy density is the key.So the question can be understood as: the current energy density of the battery why it is difficult to improve? The simple answer to the sentence is that the chemistry behind the battery limits the energy density of the battery.The energy density of the various energy carriers reproduced from the wiki.Our cell phone, tablet, notebook, watch, and well-known Tesla batteries are used in the bottom left corner of the lithium-ion battery. And then please look for gasoline, diesel, butane, propane, natural gas position. It is estimated that most people will find the following ideas:1) Battery technology is too weak2) battery technology promisingSome of the better people think about it3) Fuel cell technology will be tomorrow’s star.My idea: the above are hallucinations, hallucinations.One.the battery with the fuel behind the simple chemistryDo a little knowledge of the review (or popular).Most of the fuel and batteries we have seen in our lives, such energy carriers, are mainly related to chemical redox reactions. Energy carriers are involved in the transformation of specific chemical processes, but can always be summarized into a redox reaction.RedoxThe essence of the redox reaction is the transfer of electrons from the reducing agent to the oxidant. Do you feel like a battery? The The negative electrode of the battery is a reducing agent and the positive electrode is an oxidizing agent (not particularly accurate). Electrons from the negative through the external circuit to the cathode, and then do the work by the way: light bulbs, drive vehicles, support mobile phones and computers.Since electrons are the source of energy, then we can estimate the energy density by the density of electrons. Here we assume that the power that the electrons can do is consistent (this is clearly wrong, in fact depends on the type of oxidant and reducing agent, but if carefully examined, for the common battery and fuel, this is not the main factor).The electron density of the energy carrier, depending on the volume calculation, depends mainly on two factors;1. The volume density of the energy carrier. Solid> liquid >>>>> gas. This is a good understanding.2. The electron transfer ratio of the energy carrier. If the chemistry forget, this is very difficult to understand; if there are some impressions, this is also a good understanding. The inner electrons of the atoms do not participate in the chemical reaction, and naturally they will not be transferred. Only the outer layer will transfer the work. The electron transfer ratio is the ratio of the number of electrons involved in the reaction to the total number of molecules. In general, the number of outer electrons of the reducing agent is not so much, but the number of inner layers increases with the number of atoms increasing. More importantly, the number of atoms increases after the proton and neutrons are increasing, and both are the main source of quality.Give a few examples:1) H2-2e = 2H + hydrogen atoms only one electron, all involved in the reaction, the electron transfer ratio is 100%2) Li-e = Li + The lithium atom has three electrons, only one participates in the reaction, the electron transfer ratio is 1/3 = 33%3) Zn-2e = Zn2 + Zn atoms have thirty electrons, only two involved in the reaction, the electron transfer ratio is 2/30 = 6.7%For most substances, the proportion of electron transfer is very low, the reasons mentioned earlier. It can be seen that only the light atoms in the first two rows of the periodic table are likely to be good energy carriers. The first two elements of only 10, hydrogen helium lithium beryllium boron, carbon oxynitride. Which helium and neon are inert gases, exclusion. Oxygen and fluorine are oxidizing agents. Nitrogen is in most cases quasi-inert gas, if not an inert gas or poisonous people either smoked dead, excluded. We left five elements, hydrogen (100%), carbon (66%), boron (60%), beryllium (50%), lithium (33%).Further, if we put an atom as the negative pole of the battery. Then the energy density (mass unit) of the half-cell can be estimated by the number of electrons transferred and the atomic weight. Since then, the above ratio will be more disparate. Also take hydrogen as a benchmark:Carbon (4 / 12,33%) Boron (3 / 10.8,28%) Beryllium (2 / 9,22%) Lithium (1 / 7,14%)It is easy to find that the two elements that are most suitable for the energy carrier are carbon and hydrogen, and hydrocarbons, which are in fact the common gasoline and diesel fuels and other fuels. Car selection of these high-energy carriers as a source of energy, is already a better solution in nature. Battery with a variety of hydrocarbons compared to can be said to be inherently inadequate.Two: one of the big problems with the battery, put out the electrolyteAccording to the above explanation, we can know that the battery is difficult to exceed the fuel density in the energy density, but it seems to be able to reach half the level of fuel to 1/4 level. However, in reality the energy density of the battery is often less than 1% of the fuel. Do not believe in the data.Energy density comparison: gasoline 46.4MJ / Kg, lithium 43.1MJ / Kg, lithium battery (can not charge) 1.8MJ / Kg, lithium ion battery 0.36 ~ 0.875MJ / KgIn fact, the energy density of gasoline and lithium really much less. The main reason is that carbon to oxygen electron transfer work is not big enough (covalent bond can be different) but from lithium to lithium battery. The The The And then to the lithium-ion battery, which happened in the middle of what? TheThe reason is obvious. Lithium or lithium-ion battery inside is not only metal lithium, there are other parallel imports.I found such a formula for estimating the lithium content inside the battery. Http://www.ponytest.com/document/battery.pdfM = 0.3 * Ah. With words, the battery capacity (safety) multiplied by 30% can calculate the lithium content of the battery (g)For the well-known 18650 (cell phone notebook Tesla) battery, its weight in the 42g or so, the nominal capacity of 2200mAh or so, so its lithium content of 2200/1000 * 0.3 = 0.66g is about 1.5% of the total weight.So ah! So that we can only upgrade the lithium content of the battery can improve the energy density! TheReally so simple enough. We first look at the lithium battery in addition to lithium and what Han.Do not go! The I can not understand you can listen to it. In general, the four components of the battery are critical: the positive (discharge is the cathode), the negative (the discharge is the anode), the electrolyte, the diaphragm. Positive and negative is the place where the chemical reaction occurs, the important position can be understood. But what is the use of electrolytes? The Do not work is still very heavy weight. Then look at the map.The figure shows the battery charge and discharge process is very good. Here the first said only discharge: the battery internal, metal lithium loss in the negative electrons are oxidized to become lithium ions, through the electrolyte to the positive transfer; cathode material to be electrons are reduced, was positive lithium ion neutralization. The ideal role of the electrolyte is to transport and carry only lithium ions. Outside the battery, the electrons from the negative through the external circuit to the positive transfer, the middle of doing work. Ideally, the electrolyte should be a good carrier for lithium ions, but it must not be a good electron carrier. Therefore, in the absence of external circuits, the electronic can not be transferred from the negative inside the battery to the cathode; only the existence of external circuits, electronic transfer can be carried out.”You are not saying that” the energy carriers are involved in the process of changing the specific chemical process, but always summed up to a redox reaction. “” The essence of the redox reaction is the transfer of electrons from the reducing agent to the oxidant, “the gasoline car does not have an electrolyte The But there are electronic combustion of gasoline burning it, ye can not power it?Yes, burning must involve electron transfer, then the burning electron transfer and the electronic transfer of the battery is fundamentally different where? TheIs it orderly?Burning electron transfer is completely disordered in the microscopic category. We can not predict where the fuel and oxygen molecules will move in the direction of the next moment, we do not know the fuel on the direction of the electrons will be transferred to which oxygen molecules. The random movement of the molecules of 10 × 20-23 times with the random transfer of more electrons leads to the result of disordered energy release, or simply said, exothermic.The battery is better than the point of view. Although we still do not know the movement of each molecule inside the battery trajectory, but we can at least know: metal lithium will only lose the surface of the anode material to become lithium ions; lithium ions from the negative starting, and ultimately reach the cathode. The electrons only move from the surface of the anode material toward the positive potential of the high potential. 10 ^ 20-23 times the electrons of the co-movement, in the macro we call it the current.Sum up it In order to discharge, in order to order the electronic transfer, the battery had to carry no energy but essential electrolyte and a variety of auxiliary materials, so further reduce their energy density.Is this finished? No.Honestly this part is just a pavement.Three: the battery of the big problem, the negative surface materialHello everyone, I’m back.If you can insist on reading each line has been read here, congratulations, your understanding of the battery has been on a level.Now review the contents of the previous section. What? The All forgotten? The Not a word? The energy density of the cells is diluted due to the absence of work but essential electrolytes and the presence of other auxiliary materials.How much of these extra weights are there in the end? TheThe weight of the electrolyte typically accounts for 15% of the total weight of the battery (the link can not be found). It is estimated that the shell, external electrodes and other auxiliary materials are counted, the total weight should not exceed 50% of the total weight of the battery.Not ah, although the battery mixed with ‘water’, but also not so much water ah. The market’s lithium-ion battery energy density is also about 1% lithium. What happened to that? Why is this sentence so familiar?Drink more fresh orange, let us look at the most common lithium cobalt oxide (Tesla Roadster) electrochemical reaction.In fact, only a part of the transfer of lithium and cobalt, other elements are not involved in electron transfer.Then we make a small calculation: Elemental lithium atomic weight of 6.9, can contribute to an electronic participation in electronic transfer. The oxidant comes from the air and does not need to be considered.The total molecular weight of the reactants reacted with the lithium cobalt oxide battery was 98 + 72 = 170, but only half of the electrons were involved in the electron transfer. Because only part of the lithium atoms will react.If we think that the work of the two electrons is the same, then you can estimate the energy density of these two energy carriers ratio.Battery energy density: Fuel energy density = (0.5 /170)/(1/6.9) = 2.03% The battery is complete.Considering that the battery has half the weight of the auxiliary material, I have not counted it. So have to make a discount. The remaining 1%.So the energy density has become so: lithium 43.1MJ / Kg lithium-ion battery 0.36 ~ 0.875MJ / KgHa ha ha ha ha ha ha … … also keep up with it? The Four operations more simple ah. Now know what happened, right? TheNow do you understand why I said: The chemistry behind the battery limits the energy density of the battery.Next our question is: why the chemical reaction of the battery to be so complicated, directly reducing the energy density of the battery.This issue will be more complex, it is estimated that most people have not patience to read. So give a simple answer:For orderly.Well, no patience, you can go. The following is really long, can not read the average person.Start before the release of the picture:The rest of the students, is not that the map is very familiar with? In fact, lithium battery diagram, but this time because of the cathodic anode surface structure are displayed. Do you think they are very neat rules ah? TheNeat rules change the order, orderly.Why the positive pole of the surface structure needs to be ordered? Because it is necessary to ensure that the redox reaction occurs only at the surface of the positive and negative electrodes during charging / discharging, so that there is current.We look at the graphite (C6) where the negative.The task of the negative pole is very simple, to ensure that the discharge of lithium atoms (not ions) are lost in the negative surface of the electrons, charging them and then catch it back. Due to the low anode voltage at the time of charging, the positively charged lithium ions spontaneously move toward the negative electrode, and the electrons are returned to lithium atoms.It seems that there is no graphite thing ah? TheIf it is a one-time battery, do not need graphite. But if it is charge and discharge the battery, the anode surface material is not graphite will be other substances.Do not sell off the child, and soon in the end Editor’s Note TheHills It’s a lot of thought. When charging, lithium ions in the negative surface of the electrons become lithium atoms. and then? TheWe all know that all metals are good electron conductors, lithium is metal, so lithium is a good electronic conductor. So the first to the negative lithium atoms become part of the negative, then back to the negative lithium ions added to the ranks of the former lithium. The The TheSo that the crystal consisting entirely of lithium atoms appeared. This process, also known as crystal. The result is that the lithium crystal will pierce the diaphragm to the positive pole, so the battery short-circuit scrapped.For the crystallization of this phenomenon, we can understand so.In the charging process, we control the lithium ion is actually very weak. We can only ensure that lithium ions will move to the negative surface, but we can not guarantee that lithium ions will be evenly distributed in the negative surface. Therefore, in the absence of external constraints, the lithium crystal will be charged in the negative surface of the indefinite growth, the formation of dendrites (dendritic crystal).So there must be a constraint. To dig a pit to let lithium ions inside jump.The specific performance of this pit is the cathode surface of the graphite material. As shown in the figure above, the gap between the graphite layers is large enough to accommodate a single lithium atom, but only a single lithium atom; and then the physical adsorption between the graphite layer and the lithium atom can hold the lithium atoms, In the absence of external voltage can also be at ease when the negative surface.So, lithium atoms will not be brutal growth. But the energy density is not up.Four: the big problem of the battery three, the positive surface materialIn order to allow lithium atoms to be uniformly and uniformly distributed on the surface of the negative electrode at each charge, the surface of the negative electrode requires a solidified structure to constrain (orderly, reduce entropy) the distribution of lithium atoms. This design dilutes the energy density of the battery to a large extent.The positive electrode actually has the same problem. In order to allow the lithium ions to be uniformly and uniformly distributed on the surface of the positive electrode at each discharge, the surface of the positive electrode needs a layer of solidified structure to constrain (orderly, reduce entropy) the distribution of lithium ions The This design dilutes the energy density of the battery to a large extent.But more than thatThis is the battery cathode material charge and discharge structure changes in the diagram. Where M represents a metal atom and X represents an oxygen atom. The size of the various atoms of this figure do not take seriously. Lithium ions are much smaller than the other two.We can see that MX2 in the positive substrate on the formation of several layers of very structured (very orderly) structure, the discharge, the electrons in the positive (positive) aggregation, lithium ions move to the positive, interspersed into the MX2 structure of the gap, thus Ordered distribution in the positive surface. The metal ions in MX2 are electronically reduced, thereby acting as an oxidizing agent.Once this structure collapsed, it is impossible to reply to it.How to do? It is enough to stop Set in the battery cathode in terms of this, that is, the positive surface must maintain a certain amount of lithium ions to maintain the integrity of the structure. This amount, usually 50%.This is why the previous reaction will have an unknown amount of x. Even in the fully charged state, there are nearly half of the lithium ion to stay in the positive surface. So the energy density is lower.Off-topic: This is why the lithium battery is afraid of excessive charging, once over-charging, the cathode of the lithium-ion run, and this pile of wood will collapse.Five: the big problem of the battery four, the choice of materials on the stretched, and otherI assume that the people here are fully aware of the restrictions on the design of the rechargeable battery. In order to orderly electron transfer, in order to orderly the distribution of lithium ions and lithium atoms, the batteries need electrolytes and various auxiliary materials, the need for a regular structure on the cathode anode surface, which are at the cost of energy density.Now back to my argument:1) battery technology is too weak: how clever these designs, obviously the culmination of human wisdom.2) battery technology promising: for the future outlook, we must have a realistic attitude. Battery technology has been developed for more than 100 years, has long been the outbreak period; support the development of battery technology for the theory of physics and chemistry, their great development of the big breakthrough in the World War II is over. Foreseeable future battery technology, must be based on the current development of the battery.In the field of civilian use, the energy density of the battery is one of the most troublesome problems, but it is the most difficult problem to solve. The past battery energy density has been able to continue to improve, because scientists have been looking for elements with smaller atomic weight Acting as oxidant, reducing agent, and supporting structure. So we witnessed from the lead-acid to nickel-cadmium, from nickel-cadmium to nickel-hydrogen, from nickel-hydrogen to the current lithium-ion rechargeable battery development process, but later?Reducing agent: I said at the beginning. High proportion of electron transfer on the elements of a few: hydrogen, carbon, boron, beryllium, lithium. Which is suitable as a rechargeable battery reducing agent only lithium. Hydrogen, carbon only appears in the fuel cell. Boron, beryllium is not the main research direction, I do not know why this is.Oxidant: If you do not use transition metal, then the choice is the second line of the third line of the main group elements. Halogen is not enough, then the remaining oxygen and sulfur. The reality is that lithium air batteries (lithium oxide) and lithium-sulfur batteries have a lot of people to study, but progress is not optimistic. Why?Because the battery surface structure is a big problem.Are nanotechnology now making a lot of progress? Scientists will certainly be able to use a variety of nanowires nanotube nanospheres nano-bowl of graphene designed a fine and orderly surface structure. Those labs will be separated from each other will release a few big news ah.But there are two issues, may wish to think about.1) graphite has always been the choice of lithium battery anode material, in fact, if only consider the energy density, then the metal tin is more suitable as a negative material. But so far also sony launched tin electrode battery (Sony nexelion 14430W1) Why is this so?2) In addition to lithium cobalt oxide, the current other lithium battery cathode material is also a ternary compound Li (NiCoMn) O2 lithium iron phosphate (LiFePO4) However, due to compaction density reasons, the use of these materials, the capacity of the battery is not as cobalt lithium battery. Why do people study hard? The
Источник: Meeyou Carbide